Airborne transmission of pathogens
Table of Contents
- Table of Contents
- This page
- Books about airborne transmission of pathogens
- Review articles about airborne transmission
- Historical review papers
- Studies detecting pathogens in the air (e.g. viruses or their RNA)
- Studies where animals infected each other by airborne spread
- Other reports and discussions of airborne transmission of pathogens
- Other topics
- Long-range airborne spread
This page
This page lives at https://its-airborne.org/airborne-reference-works.
Books about airborne transmission of pathogens
List of books and review articles about airborne transmission of pathogens. Theses lists started, and for the most part still exist, as posts on Twitter and Mastodon, which can be found at the following pages. They may not be as up-to-date as this page, however.
- Books:
- Review articles:
- Moulton, Forest Ray, ed. 1942. Aerobiology. American Association for the Advancement of Science. https://www.google.ca/books/edition/Aerobiology/DNdfAAAAMAAJ
- Airborne Contagion and Air Hygiene: An Ecological Study of Droplet Infections by William Firth Wells. 1955. https://archive.org/details/airborne-contagion and https://books.google.ca/books/about/Airborne_Contagion_and_Air_Hygiene.html?id=T8nVAAAAMAAJ
- I happily present to you, kind readers, the Bible of airborne transmission of pathogens. It is on Internet Archive but also on Google Books. Click the gear icon to download it as a PDF. Interesting that I found it on Google Books, because I thought I had searched for this book there in the past and it wasn’t available for download.
- Richard L. Riley, Francis O’Grady. 1961. Airborne Infection: Transmission and Control. https://books.google.ca/books?id=qztrAAAAMAAJ
- Riley worked with Mr. Wells on aerobiology his whole life.
- Mr. Wells wrote the book I mentioned a few posts up. He was the husband of Dr. Wells, who also worked on aerobiology but passed away in the 40s. WHO. 2009. “Natural ventilation in health care for infection control”
- A World Health Organization #WHO publication, which they never mention for some odd reason. https://www.ncbi.nlm.nih.gov/books/NBK143284/.
- Kundsin, Ruth B, editor. 1980. Airborne Contagion. New York, N.Y. : New York Academy of Sciences. http://archive.org/details/airbornecontagio0000unse.
- Papers include:
- “Spread of TB via recirculated air on a naval vessel”,
- “The role of ventilation in the spread of measles in an elementary school”, and
- Langmuir, former CDC director, admitting he got airborne spread wrong but also herd immunity. What is wrong with this field, honestly. This piecs is, interestingly, not in the Langmuir collection https://hollisarchives.lib.harvard.edu/repositories/14/resources/4634/collection_organization#tree::archival_object_1289931. Hmm.
- Papers include:
- From “Airborne Contagion”:
“Airborne transmission is the most important mode of transmission of respiratory infections from person to person indoors. It may well be the most important mode of transmission for other human infections not considered as primarily respiratory. There is published evidence of droplet nuclei transmission of hepatitis B virus, smallpox, rabies, chicken pox, mumps, measles as well as tuberculosis. I am deeply grateful to Dr. Lloyd G. Herman, Dr. Richard L. Riley, and Dr. Carl W. Walter for their interest, support, and total dedication to the theme of this conference: airborne contagion.”
-
The titles of the various articles in Kundsin’s conference compilation:
- Part I. History and Epidemiology
- Historical Background. By RICHARD L. RILEY 3
- Spread of Tuberculosis via Recirculated Air in a Naval Vessel: The Byrd Study. By VERNON N. HOUK 10
- The Role of Ventilation in the Spread of Measles in an Elementary School. By EDWARD C. RILEY 25
- Changing Concepts of Airborne Infection of Acute Contagious Diseases: A Reconsideration of Classic Epidemiologic Theories. By ALEXANDER D. LANGMUIR 35
-
Part II. Epidemiology
The Epidemiology of Influenza in Humans. By MICHAEL B. GREGG 45
Epidemiology of the Common Cold. By JACK M. GWALTNEY, JR 54
Legionellosis: Evidence of Airborne Transmission. By DAVID W. FRASER 61
Legionellosis: Environmental Aspects. By G. F. MALLISON 67
Physics of Airborne Particles and Their Deposition in the Lung. By PAUL E. MORROW 71
A Tribute to William Firth Wells. By EDWARD C. RILEY 81
Part III. Bacteria as Agents of Airborne Contagion
Inhalation Anthrax. By PHILIP S. BRACHMAN 83
Aerosol Dissemination of Bacterial Plant Pathogens. By MONTY D. HARRISON 94
Airborne Spread of Brucellosis. By ARNOLD F. KAUFMANN, MARSHALL D. Fox, JOHN M. BOYCE, DANIEL C. ANDERSON, MORRIS E. POTTER, WILLIAM J. MARTONE, and CHARLOTTE M. PATTON 105
Part IV. Fungi as Agents of Airborne Contagion
Introduction. By LLOYD G. HERMAN 115
Aerial Dissemination of Fungal Spores. By DONALD E. AYLOR and PAUL E. WAGGONER 116
(Philosophical) Review of Air Currents as a Continuing Vector. By CHARLOTTE C. CAMPBELL 123
Aspergillus in Patient Care Areas. By LLOYD G. HERMAN 140
Part V. Viruses as Agents of Airborne Contagion
Viruses as Agents of Airborne Contagion. By VERNON KNIGHT 147
Aerosol Spread of Plant Viruses: Potential Role in Disease Outbreaks. By ERNEST E. BANTTARI AND JAMES R. VENETTE 167
Overview of Airborne Contagion in Animals. By LAWRENCE A. FALK, JR. and RONALD D. HUNT 174
Spread of Plant Viruses and Spiroplasmas through Airborne Vectors. By KARL MARAMOROSCH 179
Part VI. Airborne Transmission—Other Considerations
Long-Range Transmission of Bacteria. By AKE BOVALLIUS, ROGER ROFFEY, and EVA HENNINGSON 186
Surf-to-Wind Transfer of Viruses. By EDWARD R. BAYLOR and MARTHA B. BAYLOR 201
Spread of Microorganisms by Air-Conditioning Systems—Especially in Hospitals. By K. O. GUNDERMANN 209
The Role of Airborne Bacteria in the Contamination of Fine Particle Nebulizers and the Development of Noscomial Pneumonia. By STEVEN G. KELSEN and MARYANNE MCGUCKIN 218
Air Sampling in Hospitals. By DIETER H. M. GROSCHEL 230
Techniques Used for Sampling Airborne Microorganisms Associated with Industrial Clean Rooms and Spacecraft Assembly Areas. By MARTIN S. FAVERO and JOHN R. PULEO 241
Part VII. Airborne Infections in Hospitals
Documentation of Airborne Infection During Surgery. By RUTH B. KUNDSIN 255
Reduction of Deep Sepsis Following Total Hip Arthroplasty. By ROBERT H. FITZGERALD, JR 262
Ultraviolet Light for the Control of Airborne Bacteria in the Operating Room. By J. LEONARD GOLDNER, MARY MOGGIO, STEPHEN F. BEISSINGER, and DONALD E. MCCOLLUM 271
Ultraviolet Radiation and Reduction of Deep Wound Infection Following Hip and Knee Arthroplasty. By J. DRENNAN LOWELL, RUTH B. KUNDSIN, CHARLES M. SCHWARTZ, and DEBORAH POZIN 285
The Treatment of Burn Patients in a Laminar Airflow Environment. By ROBERT H. DEMLING and JEANNE MALY 294
The Contribution of A Bacterially Isolated Environment to the Prevention of Infections in Seriously Burned Patients. By GENN E. BEHRINGER and JOHN F. BURKE 300
Part VIII. Prevention and Control
Speculations on the Possible Effects of the Indoor Air on Airborne Contagion. By DONALD F. PROCTOR 308
Prevention and Control of Airborne Infection in Hospitals. By CARL W. WALTER 312
Prevention and Control of Airborne Infection in the Community. By RICHARD L. RILEY 331
Index of Contributors 341
Financial assistance was received from: • BOEHRINGER INGELHEIM LTD. • JOHNSON & JOHNSON PRODUCTS, INC. • MERCK SHARP & DOHME RESEARCH LABORATORIES • A. H. ROBBINS COMPANY • NATIONAL INSTITUTE OF ALLERGY AND INFECTIOUS DISEASES— FOGARTY INTERNATIONAL CENTER • OFFICE OF NAVAL RESEARCH
- The Air Spora. 2006 : 15–34. The Aerobiology Pathway.
- Guest Editor (s): Maureen E. Lacey and Jonathan S. West
- doi:10.1007/978-0-387-30253-9_2
- PMCID: PMC7120664
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7120664/
- 2016 Aerobiology The Toxicology of Airborne Pathogens. https://books.rsc.org/books/edited-volume/1021/Aerobiology-The-Toxicology-of-Airborne-Pathogens
- Salem, Harry, and Sidney A Katz, eds. Aerobiology. Cambridge: Royal Society of Chemistry, 2016. https://doi.org/10.1039/9781849737913-FP007
Review articles about airborne transmission
This is not all of the reviews, just the top ones. These reviews are all (mostly) written by people who work on aerosols. They are broad summaries (hence, “reviews”) of the field. Start here with your reading. You can then drill down to the actual studies if you want. That means they aren’t public health people fooling around with an air sampler they just bought and unboxed. Not joking.
- 1987 Sattar, Syed A., Mohammad Khalid Ijaz, and Charles P. Gerba. ‘Spread of Viral Infections by Aerosols’. Critical Reviews in Env Control 17, no. 2 (January 1987): 89–131. https://doi.org/10.1080/10643388709388331
- So as not to mislead you into thinking this is a recent development, I will just provide ONE reference from 1987.
- These people were never confused about 5um, etc.
-
Aerosols are dispersions in air of particles of a variety of sizes. The larger of these particles rapidly settle out, but particles of smaller size can remain suspended in air for longer periods. If the air were to be perfectly still, it would take a 100 ym diameter particle 10 sec to fall through the height of an ordinary room (about 3 m); particles with diameters of 40, 20, and 10 um would require 1, 4, and 17 min, respectively, to settle out under the same set of conditions.?? Under real conditions, the time during which aerosol particles remain suspended and the distance which they can travel from the point of their generation are greatly influenced by airflow and turbulance. Many common and natural activities in the domestic, work, or animal husbandry environments regularly result in the generation of aerosols from microbially contami- nated liquids or the resuspension in air of previously dried infectious material. For example, sneezing, coughing, and even speaking by persons carrying viruses in their mouth and respiratory tract frequently lead to the aerosolization of viruses.*$ The particles produced during sneezing and speaking (particularly when pronouncing sibi- lants) are generally larger and most of them rapidly settle out of air. Coughing, on the other hand, is known to produce more small-particle aerosols which are potentially better suited for the airborne spread of viral infections.®
- Oh, re: the lab leak issue, enjoy last line.
- Again, from 1987.
-
VI. VIRAL AEROSOLS GENERATED IN THE WORK AND HOME ENVIRONMENT The first record of a laboratory-acquired infection dates back to 1885.!! Since then, much attention has been given to the possible spread of viruses and other infectious agents to laboratory workers involved in their handling. Sulkin and Pike’** summa- rized the data from 222 reports of laboratory-acquired infections. The following types of viruses were among the infectious agents involved: eastern, western, and Venezuelan equine encephalitis; Russian spring summer encephalitis; louping ill; lymphocytic cho- riomeningitis; poliomyelitis; encephalomyocarditis; Newcastle disease; yellow fever; dengue fever; Rift Valley fever; Colorado tick fever; mumps; influenza; hepatitis; ru- bella; and agents of viral diarrhea. Analysis of the reports indicated that 30% of the infections were due to contaminated laboratory air.
-
- And exposure to air leading to infection, and filtration slashing infection. It’s all there, if you are open to it.
-
Marek’s disease Chickens Exposure to effluent air from ““donor cages’ housing 164 virus (MDV) infected animals resulted in a high incidence of MDV infection in test chicks; passage of contaminated air through certain filters partially or completely pre- vented such infection
-
- Same article, from 1987:
-
E. Lymphocytic Choriomeningitis An outbreak of lymphocytic choriomeningitis (LCM) occurred in 1972 to 1973 in personnel at a medical center in Rochester, New York.’*® Epidemiological and virol- ogical studies indicated that the source of the infection were Syrian hamsters being used there for tumor research. Cell cultures derived from these animals were also found to be contaminated with the virus. The cases of human infection were shown to occur not only through direct contact with the animals, but also from mere presence in the room where the animals were being held.
-
- and
-
… studies indicated that the source of the infection were Syrian hamsters being used there for tumor research. Cell cultures derived from these animals were also found …
-
- 2006 Tang, J. W., Y. Li, I. Eames, P. K. S. Chan, and G. L. Ridgway. ‘Factors Involved in the Aerosol Transmission of Infection and Control of Ventilation in Healthcare Premises’. The Journal of Hospital Infection 64, no. 2 (October 2006): 100–114. https://doi.org/10.1016/j.jhin.2006.05.022 or https://www.journalofhospitalinfection.com/article/S0195-6701(06)00286-6/fulltext
- 2011 By mechanical engineers in Canada. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3226423/
- 2016-09. Ijaz, M. Khalid et al and Sattar, Syed A.. ‘Generic Aspects of the Airborne Spread of Human Pathogens Indoors and Emerging Air Decontamination Technologies’. American Journal of Infection Control 44, no. 9 (September 2016): S109–20. https://doi.org/10.1016/j.ajic.2016.06.008
- Same authors as in 1980s, still writing about aerosol.
- 2019 Tellier, Raymond, Yuguo Li, Benjamin J. Cowling, and Julian W. Tang. ‘Recognition of Aerosol Transmission of Infectious Agents: A Commentary’. BMC Infectious Diseases 19, no. 1 (31 January 2019): 101. https://doi.org/10.1186/s12879-019-3707-y
- Top people
- 2020 March. Bourouiba, Lydia. ‘Turbulent Gas Clouds and Respiratory Pathogen Emissions: Potential Implications for Reducing Transmission of COVID-19’. JAMA, 26 March 2020. https://jamanetwork.com/journals/jama/fullarticle/2763852 – droplets travel further than 2 meters
- 2020 April. Bahl, Prateek, Doolan, de Silva, Chughtai, Bourouiba, and MacIntyre. ‘Airborne or Droplet Precautions for Health Workers Treating Coronavirus Disease 2019?’ The Journal of Infectious Diseases, no. jiaa189 (16 April 2020). https://doi.org/10.1093/infdis/jiaa189
- 2020 May. Anderson, Elizabeth L., Paul Turnham, John R. Griffin, and Chester C. Clarke. ‘Consideration of the Aerosol Transmission for COVID‐19 and Public Health’. Risk Analysis 40, no. 5 (May 2020): 902–7
https://doi.org/10.1111/risa.13500
- Another saying we should look at aerosol.
- 2020 June. Morawska, Lidia, and Donald K Milton. ‘It Is Time to Address Airborne Transmission of Coronavirus Disease 2019 (COVID-19)’. Clinical Infectious Diseases, 6 July 2020, ciaa939. https://doi.org/10.1093/cid/ciaa939
- 2020 June. Morawska, Lidia, and Junji Cao. ‘Airborne Transmission of SARS-CoV-2: The World Should Face the Reality’. Environ. Int. 139 (June 2020): 105730. https://doi.org/10.1016/j.envint.2020.105730
- 2020-08. Wilson, N. M., A. Norton, F. P. Young, and D. W. Collins. ‘Airborne Transmission of Severe Acute Respiratory Syndrome Coronavirus-2 to Healthcare Workers: A Narrative Review’. Anaesthesia 75, no. 8 (August 2020): 1086–95. https://doi.org/10.1111/anae.15093
- 2020 Sept. Morawska, Lidia, Julian W. Tang, William Bahnfleth, Philomena M. Bluyssen, Atze Boerstra, Giorgio Buonanno, Junji Cao, et al. ‘How Can Airborne Transmission of COVID-19 Indoors Be Minimised?’ Env Intl 142 (1 September 2020): 105832. https://doi.org/10.1016/j.envint.2020.105832
- 2020 Oct. Prather, Kimberly A, Linsey C Marr, Robert T Schooley, Melissa A McDiarmid, Mary E Wilson, and Donald K Milton. ‘Airborne Transmission of SARS-CoV-2’. Science 370, no. 6514 (16 October 2020): 303–4. https://doi.org/10.1126/science.abf0521
- 2020-11. Tang, Song, Yixin Mao, Rachael M. Jones, Qiyue Tan, John S. Ji, Na Li, Jin Shen, et al. ‘Aerosol Transmission of SARS-CoV-2? Evidence, Prevention and Control’. Environment International 144 (1 November 2020): 106039. https://doi.org/10.1016/j.envint.2020.106039
- 2021 Bourouiba, Lydia. ‘Fluid Dynamics of Respiratory Infectious Diseases’. Annual Review of Biomedical Engineering 23, no. 1 (2021): 547–77. https://www.annualreviews.org/doi/10.1146/annurev-bioeng-111820-025044
- Review from 2021, also should be in the “top” pile.
- 2021 Jan. Samet, Jonathan M, Kimberly Prather, Georges Benjamin, Seema Lakdawala, John-Martin Lowe, Arthur Reingold, John Volckens, and Linsey Marr. ‘Airborne Transmission of SARS-CoV-2: What We Know’. Clin. Infect. Dis., 18 January 2021. https://doi.org/10.1093/cid/ciab039
- 2021 April. Tang, J W, Bahnfleth, Bluyssen, Buonanno, Jimenez, J Kurnitski, Y Li, et al. ‘Dismantling Myths on the Airborne Transmission of Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2)’. J. Hosp. Infect. 110 (April 2021): 89–96. https://doi.org/10.1016/j.jhin.2020.12.022
- 2021 May. Morawska, Lidia, Joseph Allen, William Bahnfleth, Philomena M Bluyssen, Atze Boerstra, Giorgio Buonanno, Junji Cao, et al. ‘A Paradigm Shift to Combat Indoor Respiratory Infection’. Science 372, no. 6543 (14 May 2021): 689–91. https://doi.org/10.1126/science.abg2025
- 2021 May. Greenhalgh et al.‘Ten Scientific Reasons in Support of Airborne Transmission of SARS-CoV-2’. The Lancet 397, no. 10285 (1 May 2021): 1603–5. https://doi.org/10.1016/S0140-6736(21)00869-2
- 2021 August. Wang et al. ‘Airborne Transmission of Respiratory Viruses’. Science 373, no. 6558 (27 August 2021). https://doi.org/10.1126/science.abd9149
- 2021-08. Leung, Nancy H. L. ‘Transmissibility and Transmission of Respiratory Viruses’. Nature Reviews Microbiology 19, no. 8 (August 2021): 528–45. https://doi.org/10.1038/s41579-021-00535-6
- Addleman, Sarah, Victor Leung, Leyla Asadi, Abdu Sharkawy, and Jennifer McDonald. ‘Mitigating Airborne Transmission of SARS-CoV-2’. CMAJ, 1 January 2021. https://doi.org/10.1503/cmaj.210830
- Randomly found another review, discussing SARS and MERS airborne, amongst others. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7150194/#Art1
- Editorial in Indoor Air, entitled “all respiratory viruses are airborne.” https://onlinelibrary.wiley.com/doi/10.1111/ina.12937
- Tang, Tellier and Yuguo Li are all experts.
Historical review papers
- Jose-Luis Colorado et al. - And for a more comprehensive historical overview, this article spearheaded by jljcolorado is very good, I dare say. (Full disclosure: I am a co-author.)
- Randall, Ewing, Marr, Jimenez and Bourouiba. ‘How Did We Get Here: What Are Droplets and Aerosols and How Far Do They Go? A Historical Perspective on the Transmission of Respiratory Infectious Diseases’. Interface Focus 11, no. 6 (Nov 2021). https://doi.org/10.1098/rsfs.2021.0049
- Pepper, Ian L., and Charles P. Gerba. ‘Aeromicrobiology’. Environmental Microbiology, 2015, 89–110. https://doi.org/10.1016/B978-0-12-394626-3.00005-3
- But very disappointed they repeated the 5uM fiction, and the idea of droplet. So that’s a big negative. And Brankston (pic 4) is garbage. Otherwise, the chapter is a good overview of the area.
- Information Box 5.3: examples of airborne plant pathogens (and then a list of about 16 pathogens)
- Information Box 5.4: Examples of airborne animal pathogens. And then a list of about 17 items.
-
A table of aerosolized endotoxin concentrations detected downwind of biosolids operations (waste treatment facilities)
-
Influenza virus transmission among humans can occur via four mechanisms: by direct contact with infected indi- viduals: by indirect contact with contaminated objects of fomites; by inhalation of droplets that contain the virus; or by inhalation of aerosolized virus. Interestingly. despitc 70 years of research since the influenza A virus was dis- covered, ther is sill debate about the modes of influenza transmission, specifically whether influenza is mainly transmitted via true bioaerosols, or by droplets, or by direct or indirect contact (Brankston ef al 2007).
- 2022 Moreno T and Gibbons W. ‘Aerosol Transmission of Human Pathogens: From Miasmata to Modern Viral Pandemics and Their Preservation Potential in the Anthropocene Record’. Geoscience Frontiers 13, no. 6 (November 2022): 101282. https://doi.org/10.1016/j.gsf.2021.101282
Studies detecting pathogens in the air (e.g. viruses or their RNA)
Originally from https://mastodon.social/@jmcrookston/110987291726377204.
Coronaviruses
SARS-CoV-2, viable virus
- 2020-07. Santarpia et al. ‘Aerosol and Surface Contamination of SARS-CoV-2 Observed in Quarantine and Isolation Care’ Sci Rep 10, no 1 (29 July 2020): 12732. https://www.nature.com/articles/s41598-020-69286-3
- One more. Santarpia, which originally reported RNA only, then amended because they cultured viable.
- 2020-11. Lednicky, John A. et al. ‘Viable SARS-CoV-2 in the Air of a Hospital Room with COVID-19 Patients’. Intl J Inf Dis 100 (1 Nov 2020): 476–82. https://www.ijidonline.com/article/S1201-9712(20)30739-6/fulltext
- Viable SARS-CoV-2 virus from patent room in hospital in the US.
- 2020-11. Razzini, Katia et al. ‘SARS-CoV-2 RNA Detection in the Air and on Surfaces in the COVID-19 Ward of a Hospital in Milan, Italy’. The Science of the Total Environment 742 (10 November 2020): 140540. https://www.sciencedirect.com/science/article/pii/S0048969720340626?via%3Dihub
- SARS-CoV-2 RNA in hospital air, Italy. November 2020
- 2021-07. Lednicky et al., “Isolation of SARS-CoV-2 from the Air in a Car Driven by a COVID Patient with Mild Illness.” x
- 2021-11. Kotwa et al. ‘Surface and Air Contamination with SARS-CoV-2 from Hospitalized COVID-19 Patients in Toronto, Canada, March-May 2020’. J Inf Dis, 27 November 2021, jiab578.
- The conclusion of the following paper is a defensive “it’s safe for workers” article. They say b/c “ infrequent recovery of infectious virus suggests risk is limited” (they took 36 samples got 6 cultured not bad I bet, actually). However, they were able to sample live virus from air
- https://academic.oup.com/jid/article/225/5/768/6444802
- 2022-01. Ang, Alicia Xy, Irvan Luhung, Bintou A. Ahidjo, et al. “Airborne SARS-CoV-2 Surveillance in Hospital Environment Using High-Flowrate Air Samplers and Its Comparison to Surface Sampling.” Indoor Air 32, no. 1 (2022): e12930. https://doi.org/10.1111/ina.12930.
- sampled air in hospital. Found SARS-CoV-2 RNA in the air. Could not culture it, but that might have been due to the high flow rate needed to successfully capture virus in a hospital, where the air changes were already quite high. Found positive samples in a sampler 5.5 metres from the patient.
- 2023-01. Kitagawa, Hiroki, Toshihito Nomura, Yuki Kaiki, et al. “Viable SARS-CoV-2 Detected in the Air of Hospital Rooms of Patients with COVID-19 with an Early Infection.” International Journal of Infectious Diseases: IJID: Official Publication of the International Society for Infectious Diseases 126 (January 2023): 73–78. https://doi.org/10.1016/j.ijid.2022.11.003.
- 2023-03. Fortin et al ‘Detection of Viable SARS-CoV-2 in Retrospective Analysis of Aerosol Samples Collected from Hospital Rooms of Patients with COVID-19’. Clin Microb Inf (22 March 2023). - https://www.clinicalmicrobiologyandinfection.com/article/S1198-743X(23)00135-0/fulltext
- Viable SARS-CoV-2 from hospital rooms. March 2023
SARS-CoV-2, RNA only
- 2020-04-23 Park et al.
- While I am side tracked by epidemiological numbers, including attack rates, I point out that by April 2020, we had a Korean call centre experience an almost 46% attack rate for SARS-CoV-2
- 97 ppl infected, 94 were on 11th floor call centre of 216 people. 89 sympt, 4 presymp, 4 asymptomatic over 14 d quarantine. Secondary attack rate of 46%. This should have been ID doctors’ wake up call that this was airborne, but no, the denial was strong in this group.
- 2020-06. Liu, Yuan, Zhi Ning, Yu Chen, Ming Guo, Yingle Liu, Nirmal Kumar Gali, Li Sun, et al. ‘Aerodynamic Analysis of SARS-CoV-2 in Two Wuhan Hospitals’. Nature 582, no. 7813 (April 2020): 557–60. https://www.nature.com/articles/s41586-020-2271-3
- Found SARS-CoV-2 RNA in hospital air, China
- 2020-05. Chia, Po Ying, Kristen Kelli Coleman, et al. ‘Detection of Air and Surface Contamination by SARS-CoV-2 in Hospital Rooms of Infected Patients’. Nature Communications 11, no. 1 (29 May 2020): 2800. https://www.nature.com/articles/s41467-020-16670-2
- Found SARS-CoV-2 RNA in hospital air in Singapore
- 2020-11. Nissen, Karolina et al. ‘Long-Distance Airborne Dispersal of SARS-CoV-2 in COVID-19 Wards’. Scientific Reports 10, no. 1 (11 November 2020): 19589. https://www.nature.com/articles/s41598-020-76442-2
- SARS-CoV-2 RNA in Swedish hospital ventilation system.
- Over long range.
- 2021-01. López, Jorge Hernández, Álvaro Santos Romo, Daniel Coronado Molina, et al. “Detection of Sars-Cov-2 in the Air of Two Hospitals in Hermosillo, Sonora, México, Utilizing a Low-Cost Environmental Monitoring System.” International Journal of Infectious Diseases: IJID: Official Publication of the International Society for Infectious Diseases 102 (January 2021): 478–82. https://doi.org/10.1016/j.ijid.2020.10.089.
- “The current results are in accordance with those published about the search for SARS-CoV-2 in the environmental air, showing that the virus can be found in the air in the hospital zones where COVID-19 patients are placed (Faridi et al., 2020; Wang et al., 2020a, 2020b; Wu et al., 2020a, 2020b).” (López et al., 2021, p. 481)
- 2021-02. Moore et al. ‘Detection of SARS-CoV-2 within the Healthcare Environment: A Multi-Centre Study Conducted during the First Wave of the COVID-19 Outbreak in England’. J Hosp Inf 108 (1 February 2021): 189–96. https://www.journalofhospitalinfection.com/article/S0195-6701(20)30548-X/fulltext
- These authors were mostly sampling surfaces, but they found SARS-CoV-2 RNA in air. Couldn’t culture live virus from surface samples. I guess that means no touch right? 🤡🚗
- 2021-06. Horve, Patrick F. et al. ‘Identification of SARS-CoV-2 RNA in Healthcare Heating, Ventilation, and Air Conditioning Units’. Indoor Air, 29 June 2021
https://onlinelibrary.wiley.com/doi/10.1111/ina.12898
- SARS-CoV-2 RNA found in air vents.
- 2021-11. Man, P. de, et al. ‘Airborne SARS-CoV-2 in Home and Hospital Environments Investigated with a High-Powered Air Sampler’. J Hosp Inf 119 (1 January 2022): 126–31. https://www.journalofhospitalinfection.com/article/S0195-6701(21)00382-0/fulltext x
- High volume sampler used to find SARS-CoV-2 RNA in homes and hospitals.
- 2022-03. Rufino de Sousa, Nuno, Laura Steponaviciute, Lucille Margerie, et al. “Detection and Isolation of Airborne SARS-CoV-2 in a Hospital Setting.” Indoor Air 32, no. 3 (2022): e13023. https://doi.org/10.1111/ina.13023.
-
“To this end, we employed a novel electrostatic collector to sample air from rooms occupied by COVID-19 patients in a major Swedish hospital. Electrostatic air sampling in conjunction with extraction-free, reverse-transcriptase polymerase chain reaction (hid-RT-PCR) enabled detection of SARS-CoV-2 in air from patient rooms (9/22; 41%) and adjoining anterooms (10/22; 45%). Detection with hidRT-PCR was concomitant with viral RNA presence on the surface of exhaust ventilation channels in patients and anterooms more than 2 m from the COVID-19 patient. Importantly, it was possible to detect active SARS-CoV-2 particles from room air, with a total of 496 plaque-forming units (PFUs) being isolated, establishing the presence of infectious, airborne SARS-CoV-2 in rooms occupied by COVID-19 patients. Our results support circulation of SARS-CoV-2 via aerosols and urge the revision of existing infection control frameworks to include airborne transmission.” (Rufino de Sousa et al., 2022, p. 1)
-
- 2022-04. Buonanno, G., A. Robotto, E. Brizio, L. Morawska, A. Civra, F. Corino, D. Lembo, G. Ficco, and L. Stabile. ‘Link between SARS-CoV-2 Emissions and Airborne Concentrations: Closing the Gap in Understanding’. Journal of Hazardous Materials 428 (15 April 2022): 128279. https://doi.org/10.1016/j.jhazmat.2022.128279
- Tested viral load of patients as against virus in the air around those patients.
- 2022 Kurver et al. SARS-CoV-2 RNA in exhaled air of hospitalized COVID-19 patients. https://www.nature.com/articles/s41598-022-13008-4
- found SARS-CoV-2 RNA in the breath of infected individuals
- 2022 Leding et al. Detection of SARS-CoV-2 in exhaled breath from non-hospitalized COVID-19-infected individuals. https://www.nature.com/articles/s41598-022-15243-1
- found SARS-CoV-2 RNA in the breath of infected individuals
- We obtained 665 air samples from 111 included participants with confirmed SARS-CoV-2 infection. Overall, 52 individuals (46.8%) had at least one positive air sample, and 129 (19.4%) air samples were positive for SARS-CoV-2. Participants with symptoms or a symptom duration ≤ four days had significantly higher odds of having a positive air sample. Cycle threshold values were significantly lower in samples obtained ≤ 4 days from symptom onset. Neither variant of SARS-CoV-2 nor method of air sampling were associated with a positive air sample. We demonstrate that SARS-CoV-2 is detectable in human breath by electrostatic air sampling with the highest detection rate closest to symptom onset. We suggest further evaluation of the air sampling technique to increase sensitivity.
- Zhang et al. Monitoring SARS-CoV-2 in air and on surfaces and estimating infection risk …. April 27, 2022. https://www.nature.com/articles/s41370-022-00442-9
- SARS-CoV-2 was detected in the air.
- 2022-07. Parhizkar, Hooman, Leslie Dietz, Andreas Olsen-Martinez, Patrick F Horve, Liliana Barnatan, Dale Northcutt, and Kevin G Van Den Wymelenberg. ‘Quantifying Environmental Mitigation of Aerosol Viral Load in a Controlled Chamber With Participants Diagnosed With Coronavirus Disease 2019’. Clinical Infectious Diseases 75, no. 1 (1 July 2022): e174–84. https://doi.org/10.1093/cid/ciac006
- Had people live in a little modular room and measured the air.
- 2022-08. Moharir, Shivranjani C., Sharath Chandra Thota, Arushi Goel, Bhuwaneshwar Thakur, Dixit Tandel, S. Mahesh Reddy, Amareshwar Vodapalli, et al. “Detection of SARS-CoV-2 in the Air in Indian Hospitals and Houses of COVID-19 Patients.” Journal of Aerosol Science 164 (August 2022): 106002. https://doi.org/10.1016/j.jaerosci.2022.106002.
-
Taken together, we observed that the air around COVID-19 patients frequently showed the presence of SARS-CoV-2 RNA in both hospital and indoor residential settings and the positivity rate was higher when 2 or more COVID-19 patients occupied the room. In hospitals, SARS-CoV-2 RNA could be detected in ICUs as well as in non-ICUs, suggesting that the viral shedding happened irrespective of the severity of the infection. This study provides evidence for the viability of SARS-CoV-2 and its long-range transport through the air.
-
- 2022-08. Ramuta, Mitchell D., Christina M. Newman, Savannah F. Brakefield, et al. “SARS-CoV-2 and Other Respiratory Pathogens Are Detected in Continuous Air Samples from Congregate Settings.” Nature Communications 13, no. 1 (2022): 4717. https://doi.org/10.1038/s41467-022-32406-w.
- found SARS-CoV-2 RNA in school air.
- 2023-01. Gutmann et al. ‘Aerosol Measurement Identifies SARS-CoV 2 PCR Positive Adults …’. Env Res 216 (Jan 2023). https://www.sciencedirect.com/science/article/pii/S0013935122017443
- Measured SARS-CoV-2 particles out of COVID-positive and negative people. Many more out of +ve.
- Interesting:
-
“in SARS-CoV-2 PCR-positive group, 15.6% (n = 10/64) … were responsible for 64.8% of all exhaled particles counts in the group. Moreover, the 15.6%, equating to 3.5% of all patients (n = 10/288), was responsible for 51.2% of all exhaled particles.”
-
- 2021-07. Barbieri, Pierluigi, Luisa Zupin, Sabina Licen, et al. “Molecular Detection of SARS-CoV-2 from Indoor Air Samples in Environmental Monitoring Needs Adequate Temporal Coverage and Infectivity Assessment.” Environmental Research 198 (July 2021): 111200. https://doi.org/10.1016/j.envres.2021.111200.
- In the present work a short-term air monitoring was conducted at a geriatric COVID-19 healthcare facility in Trieste (Italy), where SARSCoV-2 RNA traces were detected. Evidence of viral RNA presence reported in Table 2 is coherent with those of the number of patients in the considered ward: the first day of the monitoring presents the highest number of patients and positive RNA finding in sampled indoor PM10.” (Barbieri et al., 2021, p. 4)
- Bazzazpour, Shahriyar, Masoumeh Rahmatinia, Seyed Reza Mohebbi, et al. “The Detection of SARS-CoV-2 RNA in Indoor Air of Dental Clinics during the COVID-19 Pandemic.” Environmental Science and Pollution Research International 29, no. 57 (2022): 85586–94. https://doi.org/10.1007/s11356-021-15607-6.
- Air sampling was done (n = 36) collecting particulate samples on PTFE filters at flow rates of 30 to 58 L/min. The samples were analyzed with novel coronavirus nucleic acid diagnostic real-time PCR kits. Only 13 out of 36 samples were positive for SARS-CoV-2 RNA. Logistic regression showed that sampling site’s volume, PM2.5 concentration, number of people, and number of active patient treatment units were significantly positively related with the presence of SARS-CoV-2 RNA. Thus, strategies to control the spread of COVID-19 should include reducing the number of infected people in dental clinics, adding filtration systems, and/or improving ventilation conditions.
- MacIntyre, C. Raina, Adriana Notaras, Noor Bari, et al. “Detection of SARS-CoV-2 in Aerosol and Surface Samples in High Acuity Hospital Settings during Community Epidemic Waves – Implications for Risk-Based Infection Control.” Respiratory Medicine 253 (March 2026). https://doi.org/10.1016/j.rmed.2026.108712.
- PCR only. Has a comment about fomite transmission being “prominent” which is quite surprising and I would look very closely at the 3 cited papers.
SARS-CoV-2, unsorted
- Clarke, Rachel D., Nana Aisha Garba, Manuel A. Barbieri, et al. “Detection of SARS-CoV-2 in High-Efficiency Particulate Air (HEPA) Filters of Low-Cost Air Purifiers in Community-Based Organizations.” Environmental Monitoring and Assessment 195, no. 11 (2023): 1320. https://doi.org/10.1007/s10661-023-11950-y.
- Correia, Gil, Luís Rodrigues, Mariana Afonso, et al. “SARS-CoV-2 Air and Surface Contamination in Residential Settings.” Scientific Reports 12, no. 1 (2022): 18058. https://doi.org/10.1038/s41598-022-22679-y.
SARS-1
- 2004-02. Christian, Michael D et al. ‘Possible SARS Coronavirus Transmission during Cardiopulmonary Resuscitation’. Emerging Infectious Diseases 10, no. 2 (February 2004): 287–93. https://wwwnc.cdc.gov/eid/article/10/2/03-0700_article
- It was in the room air, not because of an aerosol generating medical procedure, of course, but they wanted to reduce “panic” so they lied and said it was droplet+AGMP.
- 2004-04. Yu, Ignatius T S, Yuguo Li, Tze Wai Wong, Wilson Tam, Andy T Chan, Joseph H W Lee, Dennis Y C Leung, and Tommy Ho. ‘Evidence of Airborne Transmission of the Severe Acute Respiratory Syndrome Virus’. N. Engl. J. Med. 350, no. 17 (22 April 2004): 1731–39. https://www.nejm.org/doi/full/10.1056/NEJMoa032867
- 2004-07. Agranovski, Igor E et al. ‘Monitoring of Viable Airborne SARS Virus in Ambient Air’. Atmospheric Environment 38, no. 23 (July 2004): 3879–84 https://www.sciencedirect.com/science/article/pii/S1352231004003292?via%3Dihub
- Viable SARS-1 in air.
- Booth, Timothy F et al. ‘Detection of Airborne Severe Acute Respiratory Syndrome (SARS) Coronavirus and Environmental Contamination in SARS Outbreak Units’. J. Infect. Dis. 191, no. 9 (1 May 2005): 1472–77. https://academic.oup.com/jid/article/191/9/1472/862003
- Li, Y., X. Huang, I. T. S. Yu, T. W. Wong, and H. Qian. ‘Role of Air Distribution in SARS Transmission during the Largest Nosocomial Outbreak in Hong Kong’. Indoor Air 15, no. 2 (April 2005): 83–95. https://onlinelibrary.wiley.com/doi/10.1111/j.1600-0668.2004.00317.x
- air modelling matched the infecteds
- Amoy Gardens
- And everything written about Amoy Gardens, showing the spread between buildings, caused by negative pressure sucking air up the sewage stacks and then (a) into units (b) in plumes to buildings downwind…
IBV, a chicken coronavirus
(Not sampling, just a review article.)
“The virus spreads by both air and the fecal-oral route”

MERS
- 2014-07. Azhar, Esam I et al. ‘Detection of the Middle East Respiratory Syndrome Coronavirus Genome in an Air Sample Originating from a Camel Barn Owned by an Infected Patient’. MBio, 22 July 2014. https://journals.asm.org/doi/10.1128/mbio.01450-14
- MERS, detected in air.
- Fellow had camels. Camels caught MERS. He caught MERS from camels.
- Authors found MERS RNA in an air sample from a barn.
- They could not culture viable virus.
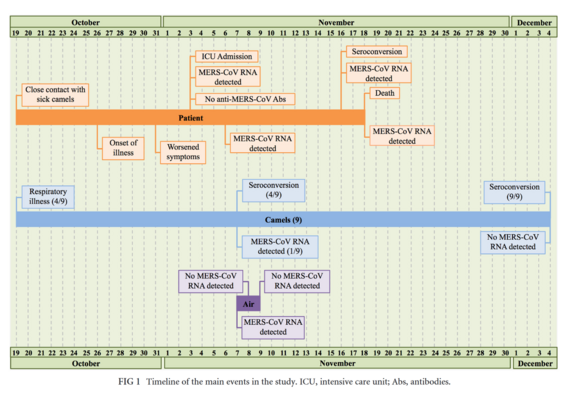
- 2014-12. Memish, Ziad A., Abdullah M. Assiri, and Jaffar A. Al-Tawfiq. ‘Middle East Respiratory Syndrome Coronavirus (MERS-CoV) Viral Shedding in the Respiratory Tract: An Observational Analysis with Infection Control Implications’. International Journal of Infectious Diseases 29 (December 2014): 307–8. https://doi.org/10.1016/j.ijid.2014.10.002
- MERS in respiratory secretions. Not in the air, but where do you think this implies it might also be, eh?
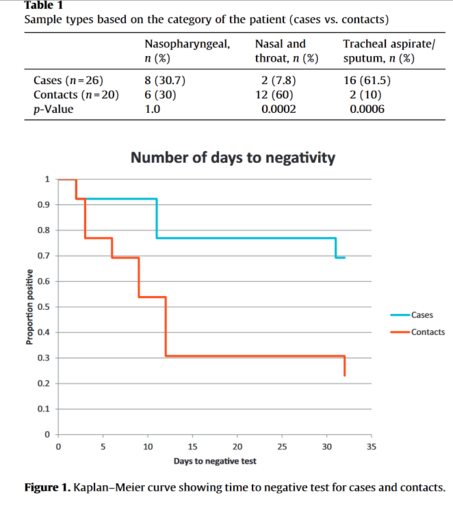
- 2016-02. Corman, Victor M., Ali M. Albarrak, Ali Senosi Omrani, Mohammed M. Albarrak, Mohamed Elamin Farah, Malak Almasri, Doreen Muth, et al. ‘Viral Shedding and Antibody Response in 37 Patients With Middle East Respiratory Syndrome Coronavirus Infection’. Clinical Infectious Diseases: An Official Publication of the Infectious Diseases Society of America 62, no. 4 (15 February 2016): 477–83. https://doi.org/10.1093/cid/civ951
- MERS in respiratory secretions #2
- “As in SARS, MERS-CoV nosocomial transmission was repeatedly ascribed to the potential of some patients to act as super-shedders or super-spreaders [6, 20]. Our analysis of viral loads, particularly in the early acute phase of disease, supports the existence of a limited number of patients with extraordinarily high viral loads. As these patients were not more likely to die of the infection, they might not have had more severe symptoms, and thus might have been able to engage in social contact despite their disease.”
- 2016-08. Kim, Sung-Han et al. ‘Extensive Viable Middle East Respiratory Syndrome (MERS) Coronavirus Contamination in Air and Surrounding Environment in MERS Isolation Wards’. Clin Inf Dise 63, no. 3 (1 August 2016): 363–69. https://academic.oup.com/cid/article/63/3/363/2595016
- Detour! Viable MERS virus in hospital air. 2016. Ya, they cultured it, you nay-saying ID doctor angling for a hospital promotion, you
other coronaviruses
- 1985 Mohammad Khalid Ijaz. Studies of the Airborne Survival of Rotaviruses and a Human Coronavirus. U of Ottawa, School of Med.
- Coronaviruses - suspended in air and viable 150 hours later. [posted July 2020]
- This thesis (1985) looked at effect of humidity & temp on aerosolized coronavirus. To keep the aerosol from depositing, put in drum rotating at 4 RPM. Not that fast.
- He cultured live virus up to 150 hours later. This is just an example of viral survival. These are Very artificial conditions. However, it demonstrates the virus can last some time in the air.
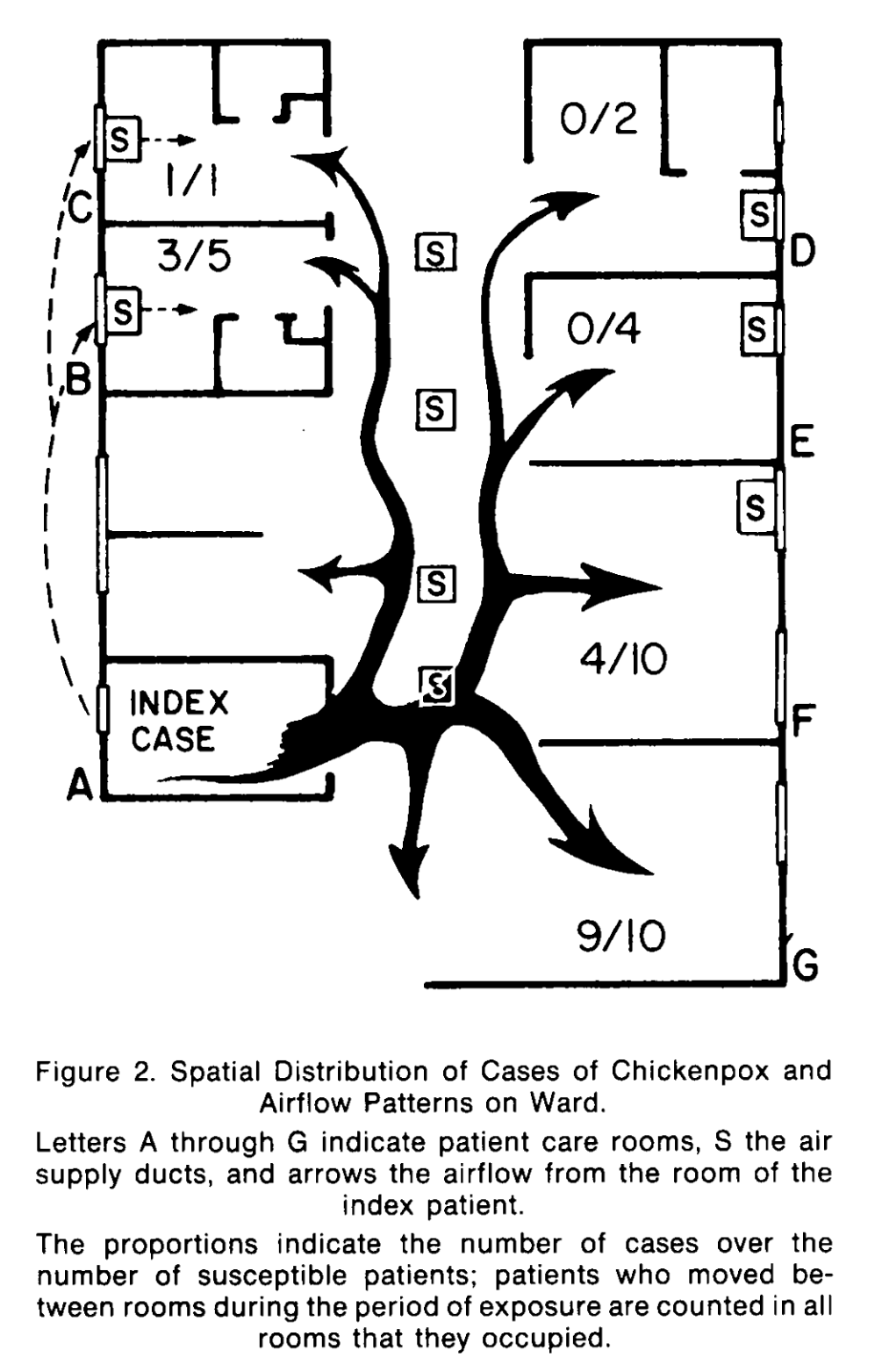
Chickenpox
- Sawyer, M. H., C. J. Chamberlin, Y. N. Wu, N. Aintablian, and M. R. Wallace. ‘Detection of Varicella-Zoster Virus DNA in Air Samples from Hospital Rooms’. The Journal of Infectious Diseases 169, no. 1 (January 1994): 91–94. https://doi.org/10.1093/infdis/169.1.91
- “VZV was detected 1.2-5.5 m from patients’ beds and for 1-6 days following onset of rash. On some occasions, VZV DNA could be detected outside the hospital isolation rooms housing patients.”
- Brankston, a very anti-airborne article, admitted airborne chickenpox had been proven, citing Sawyer and saying:
-
“If influenza could be transmitted over long distances via the airborne route, then we would have expected to review studies citing evidence similar to that reported for the varicella zoster virus and Mycobacterium tuberculosis, both of which undergo known airborne transmission.” [then citing Sawyer, above, for varicella DNA being found in the air 1.2-5.5m from beds up to 24 h later]
- Brankston also said
-
“Two epidemiological studies of varicella transmission were supplemented by airflow studies that showed directional airflow consistent with the spread of the illness.50,51”
-
- and cited these two other famous chickenpox articles:
- [50] Gustafson et al An outbreak of airborne nosocomial varicella. Pediatrics 1982; 70: 550–56. 51
- [51] Leclair et al Airborne transmission of chickenpox in a hospital. N Engl J Med 1980; 302: 450–53.
-
- [50] Gustafson described the following
- Index remained in isolation in room. 8 of 70 caught it, and seems like on a quick skim all 8 were there one afternoon. Hmm, hit rate far less than some case descriptions of COVID-19. One wonders if people are reading these old case descriptions or not.
- [51] Leclair was a report of a chickenpox patient on a ward who infected 15 others
- The introduction says chickenpox precautions are airborne, but “airborne spread has never been unequivocally demonstrated” and “Moreover, the low secondary attack rate after exposure to chicken pox in nonresidential settings suggests that airborne spread is less important than …contact.”
- “We describe an epidemic of chickenpox occurring in a hospital in which airflow studies [prove] airborne.”
Notes:
- The biggest attack rate was in room G, 9/10 people caught it - the HVAC was broken in that room.
- Room C was under strict gown and gloves precautions and patient still caught it.
- Because chickenpox, like most viruses, predominantly transmits by air.
- The rash is a red herring re touch. If throughout your body it’s in your blood and systemic. Also in saliva.
Filovirus (Ebola, etc.)
- Mekibib, Berhanu, and Kevin K Ariën. ‘Aerosol Transmission of Filoviruses’. Viruses 8, no. 5 (23 May 2016). https://doi.org/10.3390/v8050148
- This review article dealt with that question. Authors noted:
- Ebolavirus shed from mouth and nasal cavities of pigs.
- Ebola in bronchial washings of monkeys after aerosol inoculation
- Copious extracellular EBOV antigen on mucosal surfaces of the nose when exposed to infected air.
- “At the very least, the potential exists for aerosol transmission, given that virus is detected in bodily secretions, the pulmonary alveolar interstitial cells, and within lung spaces.”
- This review article dealt with that question. Authors noted:
- Ponce De Leon-Rosales, et al. ‘Ebola, Through Air or Not Through Air: That Is the Question’. Frontiers in Public Health 2 (19 January 2015): 292. https://doi.org/10.3389/fpubh.2014.00292
- Possible that the dose is very low and transmission by air might be possible even if droplet precautions are used.
- “If we consider that the infectious dose may be very low – at only 10 virus particles (3) – and that an airborne particle may contain thousands of Ebola virions, thus an exposure carried by air can be risky.”
Foot-and-mouth
- 2008 Gloster - foot-and-mouth … (talking about recovering viable aerosolized virus (viable) from pigs)
- 2008 Verreault - methods for sampling
- Christensen et al. “Detection of foot-and-mouth disease virus in the breath of infected cattle using a hand-held device to collect aerosols”. https://pubmed.ncbi.nlm.nih.gov/21723882/
- Animal studies are very useful because you can experimentally infect them. Here, they measured actual virus in cow breath.
- ““The estimated total release of airborne FMDV in 24 h from cattle is in the range of 10^4–10^5 TCID50 per 24 h depending on virus strains (Sellers and Parker, 1969; Donaldson et al., 1970; Alexandersen et al., 2002). Virus are shed in this way at a concentration of 1.0–1.5 log10 TCID50 per mL of exhaled air”
Hantavirus
- Pettersson, Lisa ‘Hantavirus RNA in Saliva from Patients with Hemorrhagic Fever with Renal Syndrome’. Emerging Infectious Diseases 14, no. 3 (March 2008): 406–11 https://wwwnc.cdc.gov/eid/article/14/3/07-1242_article
- Which other do you want me to do? For fun, hantavirus in saliva. You know what that probably means …
Influenza
- 1962 Nature.
- Flu in the air. There are probably hundreds of articles over the 20th century about air spread. There’s not a single one for droplet by the way.

- 2009 Fabian et al (Milton) - An optimized method to … (detected viable flu from air)
- Blachere, Francoise M, et al. ‘Measurement of Airborne Influenza Virus in a Hospital Emergency Department’. Clin. Infect. Dis. 48, no. 4 (15 February 2009): 438–40 https://academic.oup.com/cid/article/48/4/438/283890.
- Airborne influenza A detected in the air in emergency rooms (Blachere et al., 2009).
- Lindsley, William G. et al. ‘Viable Influenza A Virus in Airborne Particles from Human Coughs’. Journal of Occupational and Environmental Hygiene 12, no. 2 (2015): 107–13. https://www.tandfonline.com/doi/full/10.1080/15459624.2014.973113
- Influenza and viable virus again.
- Lindsley, William G. et al. ‘Viable Influenza A Virus in Airborne Particles Expelled during Coughs versus Exhalations’. Influenza and Other Respiratory Viruses 10, no. 5 (September 2016): 404–13. https://onlinelibrary.wiley.com/doi/10.1111/irv.12390
- Influenza and viable virus.
- Leung, Nancy H. L., Jie Zhou, Daniel K. W. Chu, Han Yu, William G. Lindsley, Donald H. Beezhold, Hui-Ling Yen, et al. ‘Quantification of Influenza Virus RNA in Aerosols in Patient Rooms’. PloS One 11, no. 2 (2016): e0148669. https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0148669
- Airborne influenza A detected in the air in patient rooms (Leung et al., 2016).
- 2018-01. Yan, Jing ‘Infectious Virus in Exhaled Breath of Symptomatic Seasonal Influenza Cases from a College Community’. Proceedings of the National Academy of Sciences 115, no. 5 (30 January 2018): 1081–86. https://www.pnas.org/doi/full/10.1073/pnas.1716561115
- Influenza. Found viable virus in air.
- Traxler, Selina et al ‘VOC Breath Profile in Spontaneously Breathing Awake Swine during Influenza A Infection’. Scientific Reports 8, no. 1 (5 October 2018): 14857 https://www.nature.com/articles/s41598-018-33061-2.
- Bui, Vuong N et al. ‘Bioaerosol Sampling to Detect Avian Influenza Virus in Hanoi’s Largest Live Poultry Market’. Clinical Infectious Diseases 68, no. 6 (5 March 2019): 972–75. https://academic.oup.com/cid/article/68/6/972/5088836
- “The association between positive sample types (over time and position) was strong, with 91.7% of positive OP pooled swab samples confirmed by positive aerosol samples and 81% of influenza A positive aerosol samples confirmed by positive OP swab samples.”
- Dubuis, Marie-Eve et al. ‘High and Low Flowrate Sampling of Airborne Influenza in Hospital Rooms during Three Outbreaks’. Journal of Aerosol Science 158 (1 November 2021): 10582. https://www.sciencedirect.com/science/article/abs/pii/S0021850221005553?via%3Dihub
- La Rosa et al. Viral infections acquired indoors through airborne, droplet or contact transmission
- This article refers to multiple viruses sampled out of the air. There are some refs in this article about sampling flu out of the air and flu RNA, etc.
- Influenza virus found in breath
- Found in air
- Found flu RNA in air
- Probably aint yer hands, you dirty monster bros
- Thread about this article (from 2013) is here, listing many viruses found in the air over the years, including coronaviruses, influenza, adenovirus, etc. https://mastodon.social/@jmcrookston/111994168869167484
Norovirus
- Bonifait et al. ‘Detection and Quantification of Airborne Norovirus during Outbreaks in Healthcare Facilities’. Clin Inf Dis: 61, no. 3 (1 August 2015): 299–304. https://academic.oup.com/cid/article/61/3/299/491373
- Cheng, V. C. C., S.-C. Wong, K. H. Y. Chiu, C. C. Y. Yip, S. C. Y. Wong, and K.-Y. Yuen. “Detection of Norovirus in Air Samples in a Non-Vomiting Patient: Implications of Testing Saliva for Norovirus in an Immunocompromised Host.” The Journal of Hospital Infection 103, no. 3 (November 2019): 357–58. https://doi.org/10.1016/j.jhin.2019.07.011.
- Marks, P. J., I. B. Vipond, F. M. Regan, K. Wedgwood, R. E. Fey, and E. O. Caul. “A School Outbreak of Norwalk-like Virus: Evidence for Airborne Transmission.” Epidemiology and Infection 131, no. 1 (August 2003): 727–36.
-
“As each area where a child vomited was cleaned immediately after vomiting had occurred, spread must have been by airborne transmission directly to susceptible individuals, or via contamination of the wider environment.” Marks et al., 2003, p. 733
-
“It also produced further evidence to suggest that aerosolization of virus particles can lead to direct infection.” Marks et al., 2003, p. 735
-
“this study supports laboratory evidence that cleaning with quaternary ammonium products is unlikely to alter the course of an outbreak.” Marks et al., 2003, p. 735
-
Bacteria
Francisella tularensis
- Quantification of the Relationship between Bacterial Kinetics and Host Response for Monkeys Exposed to Aerosolized Francisella tularensis. https://journals.asm.org/doi/full/10.1128/aem.01190-10
- “Francisella tularensis can be disseminated via aerosols, and once inhaled, only a few microorganisms may result in tularemia pneumonia.”
- (This is really a quantification paper. I need a quantification thread.)
- Written by Huang and Haas
Tuberculosis
- 1962-04. Riley, R L, C C Mills, F O’Grady, L U Sultan, F Wittstadt, and D N Shivpuri. ‘Infectiousness of Air from a Tuberculosis Ward. Ultraviolet Irradiation of Infected Air: Comparative Infectiousness of Different Patients’. The American Review of Respiratory Disease 85 (April 1962): 511–25. https://doi.org/10.1164/arrd.1962.85.4.511
- The famous study that showed TB was in air. Patients’ rooms connected to guinea pigs by vents. Guinea pigs would get infected over time.
- 1988-05. Kantor et al. ‘Nosocomial Transmission of Tuberculosis from Unsuspected Disease’. The American Journal of Medicine 84, no. 5 (May 1988): 833–38. https://doi.org/10.1016/0002-9343(88)90060-5
- Not about detecting it in air, but here 9 of 56 people working in a hospital caught TB from a patient who hadn’t been diagnosed with it. He seemed to infect 5 during his time on the ward before he died.
- His bacteria riddled lungs infected the next 4, in the autopsy room, when they were compressed during the post-mortem
- 2003-07. Menzies, Dick, Neill Adhikari, Marie Arietta, and Vivian Loo. ‘Efficacy of Environmental Measures in Reducing Potentially Infectious Bioaerosols During Sputum Induction’. Infection Control & Hospital Epidemiology 24, no. 7 (July 2003): 483–89. https://doi.org/10.1086/502242
- TB in air rapidly climbed when you induced sputum. Overwhelmed ventilation.
- Detected TB by plates, so cultured for all the fantastic, absolutely miserable, skeptic ID/IPAC practitioners out there.
- 2007-05. Escombe, A. Roderick, Clarissa Oeser, Robert H. Gilman, Marcos Navincopa, Eduardo Ticona, Carlos Martínez, Luz Caviedes, et al. ‘The Detection of Airborne Transmission of Tuberculosis from HIV-Infected Patients, Using an in Vivo Air Sampling Model’. Clinical Infectious Diseases: An Official Publication of the Infectious Diseases Society of America 44, no. 10 (15 May 2007): 1349–57. https://doi.org/10.1086/515397
- Transmission TB through the air from people with HIV. HIV infects the lungs too.
- 2012-05. Dharmadhikari et al. ‘Surgical Face Masks Worn by Patients with Multidrug-Resistant Tuberculosis: Impact on Infectivity of Air on a Hospital Ward’. American Journal of Respiratory and Critical Care Medicine 185, no. 10 (15 May 2012): 1104–9. https://doi.org/10.1164/rccm.201107-1190OC
- Patients wearing surgical masks lowered spread
-
“Methods: Over 3 months, 17 patients with pulmonary MDR-TB occupied an MDR-TB ward in South Africa and wore face masks on alternate days. Ward air was exhausted to two identical chambers, each housing 90 pathogen-free guinea pigs that breathed ward air either when patients wore surgical face masks (intervention group) or when patients did not wear masks (control group). Efficacy was based on differences in guinea pig infections in each chamber….”
-
“… Results: Sixty-nine of 90 control guinea pigs (76.6%; 95% confidence interval [CI], 68–85%) became infected, compared with 36 of 90 intervention guinea pigs (40%; 95% CI, 31–51%), representing a 56% (95% CI, 33–70.5%) decreased risk of TB transmission when patients used masks. Conclusions: Surgical face masks on patients with MDR-TB significantly reduced transmission and offer an adjunct measure for reducing TB transmission from infectious patients.”
- 2022-07. Dinkele et al ‘Aerosolization of Mycobacterium Tuberculosis by Tidal Breathing’. American Journal of Respiratory and Critical Care Medicine 206, no. 2 (15 July 2022): 206–16. https://doi.org/10.1164/rccm.202110-2378OC
- Tidal breathing spreads TB bacteria. And while coughs produce more TB per cough, their infrequency means tidal breathing contributes more to exhalation than cough.
strep
- 1938 . Strep in air. [originally posted Jan 13, 2021]
-
“… the fact that 22 patients..actively inspiring strep-laden air @ 13.7 cubic ft/hour… & adding to air infection by coughing & sneezing … it does not need much stretch of imagination to accept view that infection by particles in the air is no chimera.”

- Strep is a bacteria - bigger than virus. Covs are ~0.120uM. Strep is 0.5 to 2.0uM say. More than ten times bigger. Should be even more prone to “fall quickly to the ground” to people who said that.

-
other pathogens
- 2010 . Bacteria in air of turkey plant. https://pubmed.ncbi.nlm.nih.gov/20720091/
- 2011 . More pathogens in the air of a turkey plant. Authors cultured some. 2011. https://pubmed.ncbi.nlm.nih.gov/21170464/
- 2011 . Review article about airborne pathogens emitted from sewage treatment plants. https://pubmed.ncbi.nlm.nih.gov/21196384/
- Alonso et al. Evidence of infectivity of airborne porcine epidemic diarrhea virus and detection of airborne virua RNA at long distances from infected herds

Studies where animals infected each other by airborne spread
- Brockmeier, Susan L, and Kelly M Lager. “Experimental Airborne Transmission of Porcine Reproductive and Respiratory Syndrome Virus and Bordetella Bronchiseptica.” Veterinary Microbiology 89, no. 4 (November 6, 2002): 267–75. https://doi.org/10.1016/S0378-1135(02)00204-3.
-
“Experiments were designed to determine if porcine reproductive and respiratory syndrome virus (PRRSV) or Bordetella bronchiseptica could be transmitted through indirect airborne contact” ([Brockmeier and Lager, 2002, p. 267]
-
…
-
“Airborne transmission of B. bronchiseptica occurred in 5/5 trials where B. bronchiseptica was the only agent used, and in 3/5 trials where the principal pigs were coinfected with both agents. Airborne transmission of PRRSV occurred in 4/5 trials where PRRSV was the only agent used, and in 2/5 trials where the principal pigs were coinfected with both agents. Thus, airborne transmission of both agents over short distances, such as within a barn, is probable.” ([Brockmeier and Lager, 2002, p. 267]
-
- McCullers, Jonathan A., Julie L. McAuley, Sarah Browall, Amy R. Iverson, Kelli L. Boyd, and Birgitta Henriques Normark. “Influenza Enhances Susceptibility to Natural Acquisition of and Disease Due to Streptococcus Pneumoniae in Ferrets.” The Journal of Infectious Diseases 202, no. 8 (October 15, 2010): 1287–95. https://doi.org/10.1086/656333.
-
“We also did not assess the mode of transmission. It is likely that aerosol transmission was required for acquisition by contact ferrets placed 3.5 m away, because large droplets are unlikely to be capable of crossing this space, particularly in a room with such a high rate of air exchange. Sneezing by ferrets infected with influenza virus may have contributed either to formation of aerosols or dissemination across that distance. However, contacts infected with influenza virus were capable of acquiring pneumococcus from donors 3.5 m away that were not infected with influenza and were not sneezing.” ([McCullers et al., 2010, p. 1293]
-
Other reports and discussions of airborne transmission of pathogens
In the pinned posts here, https://mastodon.social/@jmcrookston/110861950615588941, you will find threads about various pathogens in the air, including:
- TB
- measles
- chickenpox
- animal coronaviruses
- rhinovirus
- filoviruses
- MRSA
- lassavirus
- leprosy
I will start moving these here.
animal coronaviruses
Are known to transmit by air https://mastodon.social/@jmcrookston/111467649735806761
filoviruses (Ebola)
https://mastodon.social/@jmcrookston/111145873500577577
influenza
https://journals.sagepub.com/doi/abs/10.3181/00379727-122-31255
Kennel cough
-
Kennel cough is a syndrome from a number of viruses. The viruses

-
Here are the viruses:
aerosol and subclinical infection

- Most vaccines non-sterilizing. Note CDV for a sec.

CDV vaccine sterilizing. Let’s see how it disseminates. Ah, systematically via lymphatic system. Thus, as I said before.
Future guesses re intranasal

Finally, the opposite of what we are currently doing re SARS-CoV-2

https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7132485/
lassavirus air
https://mastodon.social/@jmcrookston/111983739817941277
lentivirus (HIV, etc) air
https://mastodon.social/@jmcrookston/111355468559961138
leprosy (bacteria) air
https://mastodon.social/@jmcrookston/110927904297332164
MRSA (bacertia)
https://mastodon.social/@jmcrookston/112170693238203259
rabies air
https://mastodon.social/@jmcrookston/113102340965304033
RSV and B pertussis (bacteria) air
https://mastodon.social/@jmcrookston/111466006836615698
rhinovirus air
https://mastodon.social/@jmcrookston/111467417640108406
Other topics
There isn’t a single study proving droplet
See https://x.com/jmcrookston/status/1415368175592562696#m.
There is little support for fomites
A bunch of fomite articles just to see how they described fomite spread. Turns out, pretty weakly. See https://x.com/jmcrookston/status/1334851435444531200#m.
Review articles about coronaviruses
A thread with review articles about coronaviruses. They are a great place to learn all that we already knew about coronaviruses before this pandemic started. You know, from our 50 years of dealing with them. Tl;dr: most of what people “discovered” about CoVs, we already knew. Including kids, not sterilizing, persistence, in brain, etc.
https://x.com/jmcrookston/status/1310275748108922881#m
Long-range airborne spread
- Alonso, Carmen, Dane P. Goede, Robert B. Morrison, et al. “Evidence of Infectivity of Airborne Porcine Epidemic Diarrhea Virus and Detection of Airborne Viral RNA at Long Distances from Infected Herds.” Veterinary Research 45, no. 1 (2014): 73. https://doi.org/10.1186/s13567-014-0073-z.
- Hervàs, Anna, Lluís Camarero, Isabel Reche, and Emilio O. Casamayor. “Viability and Potential for Immigration of Airborne Bacteria from Africa That Reach High Mountain Lakes in Europe.” Environmental Microbiology 11, no. 6 (2009): 1612–23. https://doi.org/10.1111/j.1462-2920.2009.01926.x.
- Iddon, Christopher, Benjamin Jones, Patrick Sharpe, Muge Cevik, and Shaun Fitzgerald. “A Population Framework for Predicting the Proportion of People Infected by the Far-Field Airborne Transmission of SARS-CoV-2 Indoors.” Preprint, December 3, 2021. https://doi.org/10.1101/2021.11.24.21266807.
- MacIntyre, Chandini Raina, Arpita Das, Xin Chen, Charitha De Silva, and Con Doolan. “Evidence of Long-Distance Aerial Convection of Variola Virus and Implications for Disease Control.” Viruses 12, no. 1 (2020): 1. https://doi.org/10.3390/v12010033.
- Nagy, Alexander, Lenka Černíková, and Kamil Sedlák. “Genetic Data and Meteorological Conditions Suggesting Windborne Transmission of H5N1 High-Pathogenicity Avian Influenza between Commercial Poultry Outbreaks.” PloS One 20, no. 9 (2025): e0319880. https://doi.org/10.1371/journal.pone.0319880.
- Reche, Isabel, Gaetano D’Orta, Natalie Mladenov, Danielle M. Winget, and Curtis A. Suttle. “Deposition Rates of Viruses and Bacteria above the Atmospheric Boundary Layer.” The ISME Journal 12, no. 4 (2018): 1154–62. https://doi.org/10.1038/s41396-017-0042-4.
- Ssematimba, Amos, Thomas J. Hagenaars, and Mart C. M. de Jong. “Modelling the Wind-Borne Spread of Highly Pathogenic Avian Influenza Virus between Farms.” PloS One 7, no. 2 (2012): e31114. https://doi.org/10.1371/journal.pone.0031114.
- Van Leuken, J.P.G., A.N. Swart, A.H. Havelaar, A. Van Pul, W. Van der Hoek, and D. Heederik. “Atmospheric Dispersion Modelling of Bioaerosols That Are Pathogenic to Humans and Livestock – A Review to Inform Risk Assessment Studies.” Microbial Risk Analysis 1 (January 2016): 19–39. https://doi.org/10.1016/j.mran.2015.07.002.


![... In clinical studies, virus-laden particles within the respirable aerosol fraction have been detected in exhaled breaths of patients with influenza and in the air samples from healthcare settings during seasonal peak [9]. Moreover, the scientific literature presents evidence in support of a contribution of aerosol transmission to the spread of influenza A, including the prolonged persistence of infectious aerosolized influenza virus at low humidity; the transmission of influenza by aerosols, reproducing the full spectrum of disease, at doses much smaller than those required by intranasal drop inoculation (large droplet transmission); and the interruption of transmission of in- fluenza by blocking the aerosol route through UV irradia- tion of upper room air [9-12]. A paper by Brankston and](/pages/LaRosa-1a929b02f2695e7b.png)
![quences for hospitalized patients [14-16]. Using real-time polymerase chain reaction, Blachere and coworkers mea- sured the amount and size of airborne particles containing influenza virus in an emergency department. The authors confirmed the presence of airborne influenza virus, and found over 50% of detectable influenza virus particles to be within the respirable aerosol fraction [10]. Lindsley and](/pages/LaRosa-82c6e1854f299454.png)
![be within the respirable aerosol fraction [10]. Lindsley and colleagues detected small airborne particles containing in- fluenza RNA in a health care facility during influenza sea- son. They also found a correlation between the number of influenza-positive samples and the number and location of patients with influenza [17]. As for contact transmis-](/pages/LaRosa-9356b6152e847969.png)
![influenza season [ 18, 19]. Viruses can be transferred from surfaces to hands, and vice versa. The importance of this mode of transmission for influenza is unclear however, since, while the virus can survive on surfaces for hours or even days, it cannot survive on hands for longer than five minutes [20]. A recent study concluded that influenza A transmission via fomites is possible but unlikely to occur [19]. The overall burden of health care facility-acquired in-](/pages/LaRosa-bac75603f68ba6ed.png)